
Transit Damage Hot Roll Steel
by Barry Dillon, Forensic Services (M) Sdn Bhd, reproduced from ASM International – Practical Failure Analysis,
Hot Rolled Steel
Much of the world’s production of steel is used in the hot rolled or as-rolled condition. Hot rolled steel is one of the basic raw materials of industry and is transported great distances throughout the world. Usually there is no surface treatment (such as blasting, painting or galvanising) until it reaches its end user. Thus it is transported and stored unprotected. Inevitably incidents occur along the way and insurance claims are lodged, alleging that damage has been done. Sometimes there is no known incident, but the condition of the steel is pointed to as evidence that something must have happened.
When claims are made, adjusters and insurers need to determine some combination of the following.
- cause of damage
- timing of damage
- extent of damage
This article explains ways in which technical investigation provides answers to these questions.
Mill Scale
When steel emerges from a hot rolling mill, it is not a pretty material. At the high temperature at which rolling take place, oxidation occurs rapidly and hot rolled steel is covered with a thick dark grey material, known to the steel industry as mill scale, and to a geologist as magnetite. It is one of the forms in which iron is found in nature (Figure 1), and has the formula Fe3O4. As the name implies, it is magnetic, and is the lodestone of old, by which mariners found their way. As stated previously, most steel going under the title hot rolled steel has no additional treatment and the mill scale is left on, although occasionally some is surface treated after rolling, say by pickling in acid or grit blasting, and then left in that condition.
Mill scale is said by some to confer a degree of corrosion resistance. However it is brittle, and at some time will begin to flake off, exposing the steel, whereupon corrosion will begin. Other references will say that mill scale increases the corrosion by virtue of potential differences between it and the exposed steel. Which of these effects predominates is largely irrelevant to the gist of this article

Fig 1 Red hills of hematite in Northwest Australia. Rust is iron (steel) reverting to nature. Photograph by David Dare Parker reproduced courtesy of Australian Geographic.
Rusting
As mill scale is lost, rusting begins (see Figure 2a). The initial rust is a hydrated ferric oxide, with a formula approximating Fe(OH)3. As the rust ages the oxygen content increases, approaching the formula Fe2O3.
During this process the light orange brown colour of fresh rust darkens to a reddish dark brown (see Figures 2b and c). The geological name for Fe2O3 is hematite, and this is the most usual form in which iron is found in nature.
When steel that has been exposed for weeks to months is closely examined, it is common to see all these varieties of iron oxide. There will be mill scale, and at the edges of this scale where the steel has been freshly exposed, there will be fresh rust, alongside which older, darker rust will be apparent.
The variation is even more complicated since at any location there will variation in oxygen content and water content with depth.
However this article is about the immediately visible.
When exposed rolls are viewed from a distance the above localized variations merge to produce an even patina*. With longer exposure the patina changes in colour for the reasons given above. Examples are given in Figures 3a-b. When looking at these photographs it should be taken into account that the coils were exposed in a tropical country 6° from the equator, with year round average temperature of 27°C and 70% humidity. For purposes of comparison, an exposed test sheet is shown in Figure 3c.

Fig 2a A magnetite coating on hot rolled sheet, with fresh rust breaking through.

Fig 2b Hot rolled surface, about 60% covered with fresh rust.

Fig 2c Hot rolled steel, with all the original magnetite lost. Rust is beginning to darken where arrowed, to form hematite.
* There is a range of steels designed to never be painted, with the intent that they be used in applications where the eventual dark patina will appear as a natural effect, perhaps merging with the environment. Sometimes this works, depending on the skill of the architect.

Fig 3a Hot rolled coils after exposure for 2-4 weeks and 10 weeks in Malaysia.

Fig 3b Hot rolled coils after exposure for 34 weeks in Malaysia.
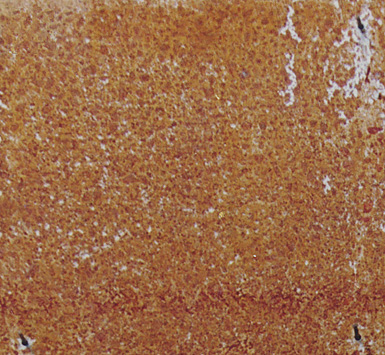
Fig 3c Corrosion in 10 days on clean, descaled steel exposed outdoors in Malaysia.
Insurance of Hot Rolled Steel
Given that rusting begins when the steel is rolled, and is a natural, inevitable process in which the iron is simply reverting to nature, it is curious that this material should be insured by policies that sometimes include the words rust, oxidation and discolouration. The difficulty presented by the presence of these words is highlighted further when a study of product standards for hot rolled steel reveal the absence of these same words. In fact, to a metallurgist, it is odd that there should be any claims at all for the rusting of hot rolled steel.
The inappropriate wording of such policies is further highlighted by the fact that hot rolled steel is never wrapped for protection and is commonly stored outdoors. Clearly, from the background given above, every shipment of hot rolled steel coil and plate is rusted to some extent. The questions are how much, and is the rusting logically claimable under an insurance policy. We are often asked to provide qualitative data and opinion to enable an adjuster to interpret the policy.

Fig 4 Coil Storage : These coils weighed over 20 metric tons each and when placed on reclaimed land tended to sink, despite stones placed beneath. The land was subject to flooding. Some coils were placed on top, causing the ones beneath to sink further into the ground. Tarpaulins were placed over others, ensuring moist conditions round the clock. The insurance contract covered up to 60 days storage after the unloading from the vessel.
For an article devoted to damage during transit there might seem to be undue emphasis on deterioration that occurs during land storage.
On many claims we have been consulted on, the sea voyage occupied only a small proportion of the insurance interval. The reason for this is the wide range of time intervals covered by marine policies.
These can cover the voyage itself, or might extend for long intervals on either side, during which the coils are simply sitting outdoors, sometimes in abysmal conditions (see Figure 4).



Fig 5 Pipe Storage : The use of end caps on these pipes did not go well with outdoor exposure. Any water entering could not escape, resulting in a humid atmosphere inside, with upper pipe temperatures reaching 100ºC in the sun. This resulted in the loss of mill scale and corrosion over the entire inside circumference. (a) Normal mill scale finish in pipe where no water had entered; (b) plastic end caps; (c) water had entered this capped pipe, with the resulting humidity causing corrosion that lifted the mill scale.
Significant factors and mechanisms applying to corrosion of hot rolled steel stored outdoors in the tropics are as follows.
- Tropical countries tend to have high rainfalls, but the proportion of time it is raining can be low. An exposed surface can be wet for less than an hour a day, whereas a surface in a situation where the water cannot escape, eg between the coiled sheet, will be wet for 24 h/d. The regularity of the rainfall ensures some surfaces never dry out.
- It is well known that with steel piles in water, corrosion is greatest at the splash zone, partly because of the optimum combination of wetting and availability of oxygen. Much the same applies to tropical corrosion where coils sit in pools of water (see Figure 6). At many of the storage sites we have visited, corrosion would have been less had the steel been totally submerged.

Fig 6 Coils are sometime stored outdoors in poor conditions.
- The initiation of corrosion and the accumulation of scale on surfaces leads to an additional acceleration beneath continually-wet deposits as a result of electrochemical effects (see Figures 7a, b, and c).
Fig 7 Rail Storage : Steel rails for a long term project were imported into Malaysia and stored in the open. A large proportion were placed in one stack 6m by 6m by 5m high. The rails from this and other stacks were used progressively, being taken from the tops of the stacks. After 17 months it was declared that the condition of the remaining rails in the large stack was so poor they were no longer acceptable. The rails in the smaller stacks were acceptable. Investigation revealed the size of the stack prevented the rails from drying out. Occasional rain, typically for a short time each day, kept the inside of the stack perpetually moist. Falling rust accumulated on horizontal surfaces, leading to deep pitting under the deposit.


(7a) Fallen rust accumulated on surfaces within the stack.
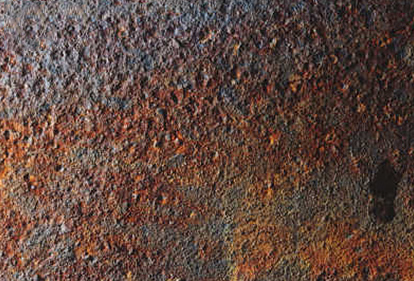
(7b) Typical corroded upper surface from small stack.

(7c) Typical corroded upper surface from a large stack.
- The average 24hr, year round temperature of a country like Malaysia, close to the equator, is of the order of 27°C. This compares with say Zurich at 9°C, London at 11°C, and New York at 12°C. Corrosion is a chemical reaction and other factors being equal, the higher the temperature the faster the reaction.
Failure Analysis
Shipment History
The raising of an insurance claim many months after the goods left the manufacturer can result in obvious difficulty in determining how and when damage occurred. The unraveling of such claims can involve a trail of paperwork extending across the globe.
When we are appointed we immediately request full details of the shipment history and purchase details. Sometime this reveals the steel had been made long ago and had been sitting around long before the insurance began.
Fig 8 Contamination by Acid : In this shipment from Europe, a survey prior to loading revealed seepage and a positive result for a chloride spot test. Note that these coils had been descaled. When the coils arrived in Malaysia six weeks later, there was unusually severe corrosion in the same areas of the coils. It was most probable the coils still conformed to the original specification, but the damage was then compounded by months of storage on swampy land, with tarpaulins holding moist air about the coils. Chemical analysis of the corrosion product revealed high fluoride levels. In retrospect, the positive test in Europe was probably from a pickling solution with HF content that had not been washed off.

(a) The application of a silver nitrate swab produced a white precipitate, usually indicative of the presence of chloride ions. Courtesy of Unilex Maritime s.a.r.l., France

(b) Taken six weeks after survey in Europe. Courtesy of Link Survey (M) Sdn Bhd, Malaysia
A common practice is to employ surveyors at various stages during transport, such as unloading and unloading. However at each inspection, the surveyor has only limited information and often the problem is not apparent until all the documentation is collected and studied (see Figure 8). Individual surveyors enroute are unlikely to know the details of manufacture, the end application or the applying standards of acceptance and rejection.
For example, delivery documents might indicate an international standard under which the steel was purchased, with no mention of separate agreements between importer and their client that place more onerous restrictions on dimensional variation.
Has Significant Corrosion Occurred?
In addition, as mentioned previously, all hot rolled steel is rusted to some extent, so the question of how much rust is acceptable, or even reportable, is a matter of diverse opinion. Non-destructive testing can be used to quantitatively describe the dimensional condition of the shipment. Many adjusters are surprised when we point out that the shipment still conforms to the standard under which it was purchased. This is because a feature of rusty steel is that damage can look far worse than actual. When steel corrodes, the oxide is bulky, commonly occupying a volume many times that of the steel that was converted into rust. Thus the bulky oxide gives a false visual impression of the quantity of steel lost. In reality the standard specifications for hot rolled steel, from whatever origin, provide generous allowances for deviation about a nominal thickness, whether in terms of thickness or weight. In addition, the rusting of the steel and the lifting of the mill scale can render subsequent abrasive blasting easier, rather than increase costs as is sometimes claimed.
From the many investigations we have carried out, we offer the following truism, which we do not claim to be an infallible rule, but in our opinion almost always holds for hot rolled steel.
In a normal voyage, wetting by itself will not result in such corrosion that hot rolled steel no longer conforms to the standard to which it was manufactured.
This leads to the following corollary.
If hot rolled steel from a voyage is found to have corroded to the extent that it no longer conforms to the standard under which it was manufactured, and there is no contamination other than wetting, then much of the corrosion had occurred prior to or after the voyage (see Figure 9).

(a) Steel from Poland claimed to have been corroded during a voyage.
Fig 9 Prior Corrosion : Examination of this shipment of steel from Poland revealed that the corrosion was so severe it could not have occurred during the voyage. That is, the corrosion rate would have been in excess of that possible, even if it had been wet for the entire voyage. It was found that the steel had been stored under good conditions during the voyage and had not been wet.
There were no other materials in the hold capable of accelerating corrosion. Inspection was carried out soon after arrival and storage corrosion after the voyage could not have been a factor. It was clear that the steel had been exposed to the elements for a long time prior to the voyage.

(b) Feature of this corrosion was that the mill scale had lifted on all surfaces, irrespective of orientation.

(c) Severe corrosion in the channel, to the extent that it could not have occurred during a single voyage.
Manufacturing Defects
Examination of shipments can reveal that there are problems that originate in manufacture. We often suspect the insured is well aware of this, but finds it simpler to claim for corrosion damage in his own country than to attempt to recover from a manufacturer on the other side of the world. Figures 10 – 12 illustrate problems that have their origin in manufacture.

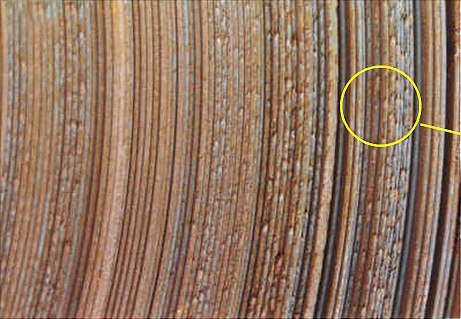

Fig 10 Prior Defects : The explosion in world trade has increased the choices of sources of steel available to a manufacturer. There can be considerable saving in buying from a non-traditional source. However the more the sources the greater the possible range of defects. Steel from non-traditional sources can exhibit a range of quality problems which can complicate an insurance claim. For example, on a single shipment, there might be an unusual severity of rusting, together with serrated edges, coiling damage, and handling damage caused by poor strapping. (a) Burred edges on hot rolled coil, caused by rubbing during coiling. Such damage is not possible during transport; (b and c) serrated edges on hot rolled steel coil. This condition is a natural result of hot rolling, but it should be trimmed away at the steel mill prior to shipping.



Fig 11 Strapping : Strapping can be poor to begin with, or can deteriorate with handling and/or corrosion. If straps are unevenly tensioned, there will be excessive stress on the tightest. In turn each will fail. Straps are made of thin steel and a little corrosion can significantly weaken them. A tightly wound, tightly strapped coil is extremely strong, with each widing supported by its neighbors. However, once a coild is loosened and windings telescope, they are very vulnerable to damage. Unwinding alone, with no damage, can result in rejection because the coil will not fit into pipe making equipment and there is no way to recoil. (a) Edge damage. The orange color testifies to recent occurrence; (b) a coil with straps beginning to fail; (c) a coil with all straps broken, allowing the coil to unwind every time it is handled.


Fig 12 Poor Strapping : (a) Coils of hot rolled steel from Russia, said to have suffered damage during handling during the voyage. Examination revealed that the damage had occurred during transport, but the coils had been rendered vulnerable to damage because of poor strapping at manufacture. The loose strapping resulted in the coils tending to unwind, with breaking of straps and further loosening. The coils were then able to telescope, and every time they were lifted with slings, there was edge damage (b).
When did the Corrosion Occur?
The most common question we are asked is when did the corrosion occur. The interest of the adjuster/insurer is whether the corrosion occurred during the period of insurance. Unfortunately the use of dating techniques in archeological studies has give the false impression to many that rust can be quantitatively dated. The colour of the rust does sometimes allow us to express an opinion, provided we are consulted quickly.
For example, if we examined a shipment a few days after arrival in Penang from a 10 day voyage from India and saw an even patina of dark red/brown rust, we would say that the corrosion did not occur on the voyage. If we were first asked to examine the same shipment two months later after outdoor storage, we would not be able to provide the same opinion by observation alone
The magnitude of the corrosion might also allow the question to be determined. If analysis shows no contaminating species and we are satisfied that wetting alone could not have resulted in the metal loss, we would conclude that corrosion occurred before the voyage. To make such a judgment it is necessary to know the nature of the other cargo on the vessel. Were there any materials that might have accelerated corrosion? Was there machinery that might have been expected to suffer damage, had the environment been unusually corrosive?
Corrosion from Contamination
In our experience when abnormal corrosion has occurred during a voyage, it is a result of contamination. Examples of contaminants are seawater, fertilizer and acid (see Figures 8, 13 – 14). Even if the shipment still conforms to specification, a proven incidence of contamination during the voyage and abnormal corrosion will provide an insured with a strong case and the problems have to be addressed rather than dismissed. It is a fact that very rusty steel looks bad, and can be more difficult to sell, even though it conforms to specification. However it is difficult to justify the scrapping of hot rolled steel that still conforms to specification, irrespective of how it looks. See Figure 13 for a typical resolution. Knowledge of the other cargo and chemical analysis of corrosion products are necessary steps in failure analysis.



Fig 13 Contamination by Seawater : The steel plates shown here (a) were carried on a ship which caught fire off Singapore. The fire was fought with seawater, and by the time the plates were removed there was very visible corrosion (see far right) and a claim was made for 80% damage allowance. It was determined that despite the long contact with seawater, the steel conformed dimensionally to the product standard under which it had been purchase. The plates were decontaminated and surface treated, at a cost of about 20% of the value of the shipment. The steel plate on the left (b) has a ‘normal’ appearance, being mainly covered with mill scale. The plate on the right (c) has been in a ship while contaminated with seawater. While it looked bad, it still conformed to the original specification.
Fig 14 Contamination by Fertilizer : This shipment of hot rolled pipe exhibited unusual corrosion upon discharge. Inquiries revealed there were fertilizer (ammonium chloride) and steel-making additions stored above the pipe in the hold. The porous, granular steel-making addition struck to the pipe, providing a reservoir for the fertilizer and moisture. It was likely that most of the corrosion occurred after the voyage while the pipes were stored outdoors and the insurance inquiries were being carried out.
Corrosion pitting inside of pipe, revealed after removal of porous steel-making material seen below.
Steel-making addition that had been lying inside the pipe. Found to be moist and contaminated with ammonium chloride.
Summary
The investigation of alleged corrosion damage to hot rolled steel during transit require metallurgical, chemical and corrosion knowledge. Familiarity with non-destructive techniques and sampling procedures are of assistance. A complete record of shipment history is also required, including the purchasing specification and observations and photographs taken during surveys enroute. A frequent conclusion of such investigations is that the alleged corrosion is of no significance or did not occur during the voyage.
Need clarity on a large and complex incident?
Our forensic consultants are ready to investigate, analyse and support you – from claims to courtrooms.
